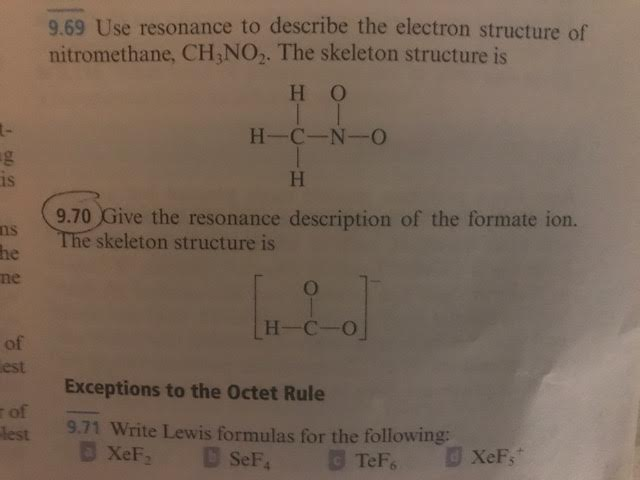
Use Resonance to Describe the Electron Structure of Nitromethane
Use resonance to describe the electron structure of nitromethane CH3NO2. So rezoning structures for this molecule is this is Method group and here this is nitro group born with oxygen in double bond with oxygen and Arizona is represented by double.

Devens Gust S 364 Research Works In Entertainment And Arts And History
Use resonance to describe the electron structure of nitromethane CH3NO2.

. The skeleton structure is. It exists in this state. Before investing in a foreign location a firm must consider three things.
XeF 5 967 Write Lewis formulas for the following. Draw the Lewis structure of nitromethane CH3NO2 clearly indicating resonance contributors as well as non-bonding pairs of electrons and formal charges as relevant. Draw the curved arrows that show conversions from one resonance structure to another.
The skeleton structure is. Nitromethane is said to be a resonance hybrid of these two structures. The two effects add together to increase the dipole moment of nitrobenzene relative to nitromethane inductive.
The citizens of Paris were terrified during World War I when they were suddenly bombarded with. Finally draw a resonance hybrid for nitromethane CH3NO2. This preview shows page 5 - 9 out of 17 pages.
Describe how comparing the anatomy of living species provides evidence of evolution. 16 are fictitious but nitromethane is a real molecule. Before investing in a foreign location a firm must consider three 1.
Add curved arrows to both resonance structures of nitromethane to show the delocalization of electron pairs. Resonance structures are necessary because of the inadequacy of a single Lewis struc- ture to represent nitromethane accurately. Question 6 1 point When one use bond-line structure to describe CHzCHCH2 one can draw structures A Band Cas well as D Which structure can be used to best describe the charge delocalization property of CHzCHCHz and why.
Nitromethane ch3no2 burns in air to produce significant amounts of heat. 100 3 ratings Transcribed image text. Use resonance to describe the electron structure of nitromethane mathrmCH_3 mathrmNO_2.
Because we have no way to describe nitromethane accurately with a single Lewis structure. Use resonance to describe the electron structure of nitromethane CH3NO2. Structure you drew in part A.
Nitromethane is said to be a resonance hybrid of these two structures. The skeleton structure is. An electron in a hydrogen atom is in a state with l.
The double-headed arrow SLLA means that nitromethane is a single compound that is the average of both structures. Science Chemistry QA Library A. Use resonance to describe the electron structure of nitromethane CH 3 NO 2The skeleton structure is.
BCl 3 b. There can never be more than two resonance structures for any molecule. Use resonance to describe the electron structure of nitromethane.
The carbon position arc labeled from 1-3. The skeleton structure is. Use resonance to describe the electron structure of nitromethane CH3NO2.
Resonance structures are necessary because of the inadequacy of a single Lewis struc- ture to represent nitromethane accurately. Draw curved arrows to show how this can be converted to the Lewis arrows to show how this can be converted to the resonance structure given. The skeleton Use resonance to describe the electron structure of nitromethane CH3NO2.
The skeleton structure is H C N H H O O 965 Write Lewis formulas for the following. The skeleton Use resonance to describe the electron structure of nitromethane CH3NO2. Select Draw Rings More Erase Select Draw Rings More Erase N N 0.
2 Only ionic compounds use resonance structures 3 The real structure of a molecule is an average or. Resonance structures are necessary because of the inadequacy of a single Lewis struc-ture to represent nitromethane accurately. Nitromethane CH3NO2 CID 6375 - structure chemical names physical and chemical properties classification patents literature biological activities safety.
We review their content and use your feedback to keep the quality high. The skeleton structure is. Give the resonance description of the formate ion.
The skeleton structure is. The skeleton structure is. The skeleton structure is.
Phosphorus pentachloride is normally a white solid. General Chemistry 11th Edition Edit edition Solutions for Chapter 9 Problem 69QP. Use resonance to describe the electron structure of nitromethane CH3NO2.
We will write the reason instructor for C S three and or two. Use resonance to describe the electron structure of nitromethane CH3NO2. Blue orbital and the adjacent red orbital orbital is 0o.
Draw the resonating structure of Nitromethane - Chemistry - Organic Chemistry Some Basic Principles and Techniques. 961 Write resonance descriptions for the following. Draw the major resonance structure then draw curved B.
For each resonance structure assign formal charges to all atoms that have formal charge. The costs benefits and risks political economic or legal associated with entering into a. Use resonance to describe the electron structure of nitromethane.
Nitromethane sometimes shortened to just Nitro is an organic compound with the chemical formula CH3NO2. The double-headed arrow SLLA means that nitromethane is a single compound that is the average of both structures. The skeleton structure is.
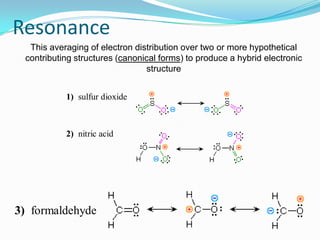
The two resonance structures in Eq. There are a total of 24 valence electrons in the NO 2 Cl Lewis structure. The actual structure of nitromethane is a resonance hybrid of the two canonical forms I and II.
Use resonance to describe the electron structure of nitromethane CH3NO2. The skeleton Use resonance to describe the electron structure of nitromethane CH3NO2. Thionitromethane is redrawn for you.
SO 3 963 Use resonance to describe the electron structure of nitromethane CH 3 NO 2.

Ch3no2 Lewis Structure How To Draw The Lewis Structure For Ch3no2 Youtube

Oneclass Use Resonance To Describe The Electron Structure Of Nitromethane The Skeleton Structure

Ionic Bond Definition Example Properties And Formation Condition Ionic Bonding Organic Chemistry Books Electron Affinity

Lewis Structure Chlorine Chloride Electron Diagram Png Clipart Atom Black Black And White Brand Calcium Chloride Free Png Download

Electronic Structure Of Ch 3 No 2 2 A ј As Predicted At The Ub3lyp Download Scientific Diagram
Solved Use Resonance To Describe The Electron Structure Of Chegg Com

An Introduction To Reactive Oxygen Species Measurement Of Ros In Cells Reactive Oxygen Species Oxygen Species

Draw An Electron Dot Diagram To Show The Formation Of Each Of The Following Compounds Methane

Ch3no2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
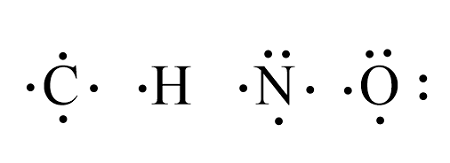
Draw And Explain The Lewis Diagram For Nitromethane Ch3no2 Study Com

What Is Synthesis Chemical Reaction In Chemistry Chemical Reactions Organic Chemistry Books Chemistry

Oneclass Use Resonance To Describe The Electron Structure Of Nitromethane The Skeleton Structure

Use Resonance To Describe The Electron Structure Of Nitromethane The Skeleton Structure Oneclass

Bringing Out The Potential Of Organoboron Compounds By Designing The Chemical Bonds And Spaces Around Boron Chemical Communications Rsc Publishing Doi 10 1039 D2cc00653g
Electron Structure And Optical Properties Of Conjugated Systems In Solutions Springerlink

Draw And Explain The Lewis Diagram For Nitromethane Ch3no2 Study Com

Comments
Post a Comment